News
What Is Minimally Invasive Surgery (MIS)? Devices & Disposable Systems Overview
What Is Minimally Invasive Surgery (MIS)? Devices & Disposable Systems Overview
A comprehensive overview of minimally invasive surgery (MIS), including laparoscopic access devices, fluid and gas management systems, disposable surgical consumables, and OEM sourcing considerations for global medical distributors.
What Is Minimally Invasive Surgery (MIS)?
Minimally invasive surgery (MIS) refers to surgical techniques performed through small access ports using specialized instruments and visualization systems. Unlike traditional open surgery, MIS relies on laparoscopic devices, trocars, insufflation systems, and disposable surgical consumables to support controlled procedural workflows.
MIS is widely adopted in general surgery, gynecology, urology, and abdominal procedures, where standardized laparoscopic systems are integrated within modern operating rooms.
Core Device Categories Used in MIS
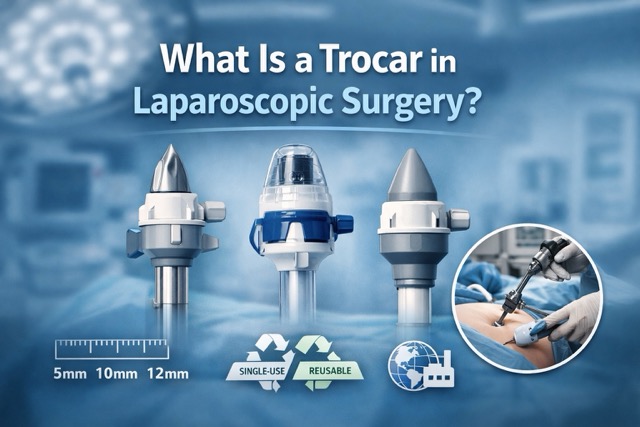
Laparoscopic Access Devices
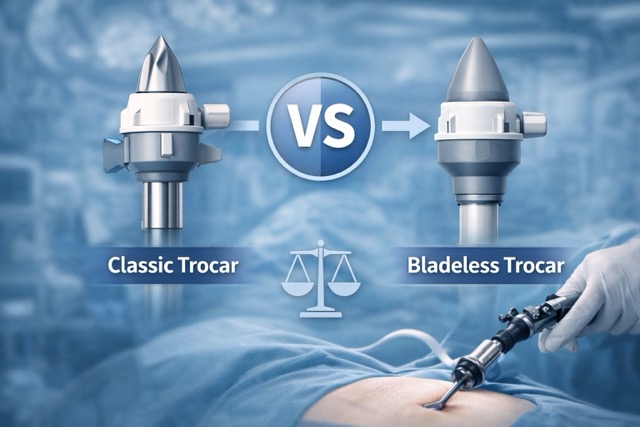
- Trocar Essentials
- Optical and classic trocars
- Access port systems
- Instrument compatibility (5mm / 10mm / 12mm)
Fluid Management Systems
- Laparoscopic Suction Irrigation Systems
- Disposable irrigation tubing sets
- OR suction integration
- Controlled fluid delivery and removal
Gas Management & Filtration
- Laparoscopic Filtration Systems & Tubing
- Insufflation filters
- CO₂ insufflation compatibility
- Disposable filtration units
Specimen Retrieval & OR Safety
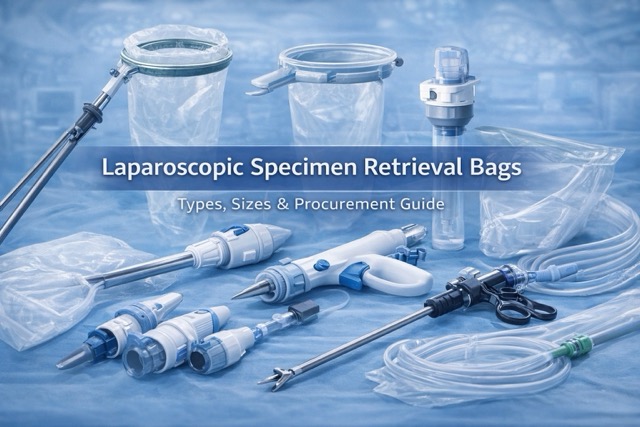
- Specimen Retrieval Bag Series
- OR Essentials
- Sharps management systems
- Disposable protective covers
Disposable Surgical Consumables in MIS
- Single-use sterile packaging
- Standardized operating room integration
- Reduced reprocessing workflow
- Traceable SKU documentation
- CE MDR and ISO 13485 compliant manufacturing
Many modern MIS workflows rely on disposable surgical consumables to support infection control policies, predictable cost structures, and streamlined supply chain logistics.
How Global Buyers Evaluate MIS Device Manufacturers
- OEM / ODM production capability
- Regulatory compliance (CE MDR / ISO 13485)
- Compatibility across laparoscopic platforms
- Private labeling programs
- Export documentation and packaging readiness
- Long-term supply chain stability
Frequently Asked Questions About MIS
What does MIS stand for in surgery?
MIS stands for Minimally Invasive Surgery, a technique using small access ports and specialized laparoscopic devices.
What devices are commonly used in minimally invasive surgery?
Common devices include trocars, suction irrigation systems, insufflation filters, specimen retrieval bags, and OR disposable consumables.
Are MIS devices typically disposable?
Many modern MIS workflows incorporate disposable surgical consumables to align with infection control standards.
Can MIS consumables be supplied through OEM programs?
Yes, many manufacturers provide OEM and private labeling programs for global medical distribution.
OEM Minimally Invasive Surgery Consumables Manufacturer
As a professional laparoscopic and MIS disposable manufacturer, we provide OEM and ODM surgical consumables for global distributors.
Our portfolio covers laparoscopic access systems, fluid management devices, gas filtration systems, smoke evacuation sets, specimen retrieval bags, and OR safety consumables designed for standardized operating room workflows.
We support scalable production capacity, CE MDR and ISO 13485 compliance, private labeling programs, and export-ready packaging solutions for international medical device markets.