About Us

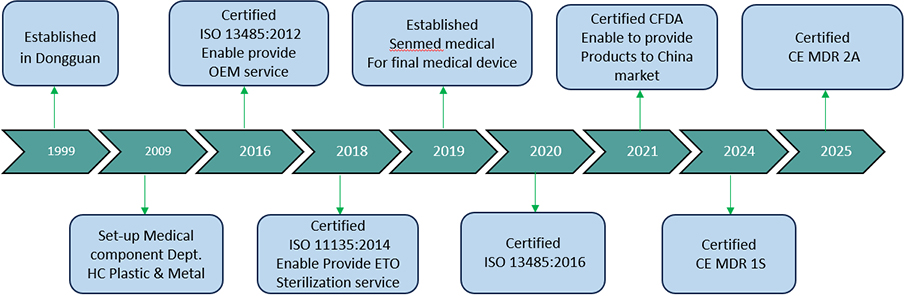
Senmed Medical is a certified manufacturer specializing in high-quality disposable surgical consumables. With over 20 years of experience in medical materials, precision engineering, and cleanroom production, we deliver reliable and cost-effective products for global operation room needs.
Our vertically integrated production model enables stable supply, full traceability, and flexible OEM/ODM services for healthcare distributors and medical device brands worldwide.
Our Commitment:Building long-term trust and enabling mutual growth with our partners.


We supply a focused range of disposable surgical consumables:
- Suction & Irrigation Systems
- Surgical Filters & Accessories
- OR Equipment Covers
- Safety Solutions (Sharp boxes, Sharp pads)
We also offer OEM/ODM customization, including material selection, mold development, and private labeling.
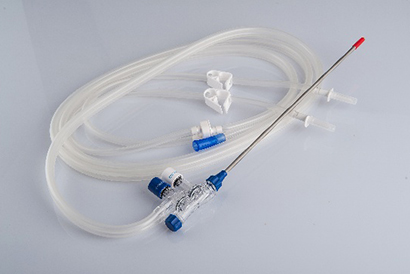


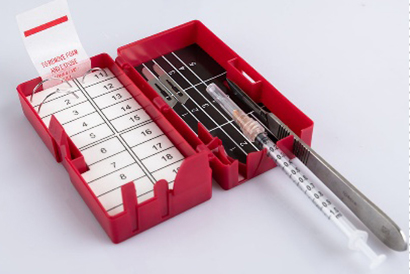
Our end-to-end, in-house production ensures consistent quality and cost-effective delivery.
- In-house R&D & DFM engineering
- Precision mold development
- Injection, extrusion & assembly in Class 100,000 cleanrooms
- EO sterilization compliant with ISO 11135
- Internal laboratory for testing & validation
This integrated model shortens lead times and supports stable, high-volume production.




Our facility is designed for scale, precision, and international compliance.
- 9,500 sqm manufacturing area
- 120 employees including dedicated R&D and QA teams
- Class 100,000 cleanroom production
- Filters with Tubing — 1,000,000 sets
- Suction & Irrigation Sets — 400,000 sets
- Light Handle Covers — 1,500,000 pcs
- Sharp Boxes & Pads — 1,500,000 pcs



We operate under a strict quality management system with full traceability from raw materials to finished goods.
Certifications:




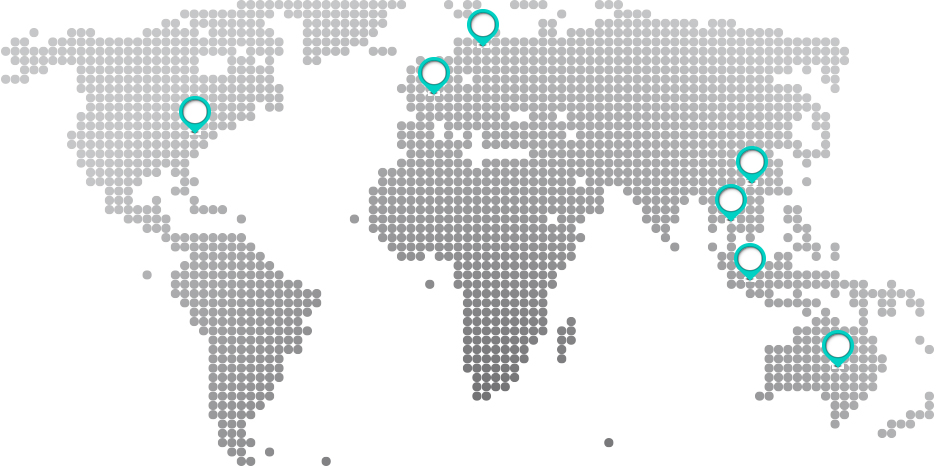
Senmed supplies surgical consumables to distributors and OEM partners across major international markets, including:
UK, France, United States, Australia, Thailand, Malaysia, and China.
We are committed to delivering precise, reliable, and cost-effective surgical consumables.
Contact us to discuss your OEM/ODM project or distribution needs.