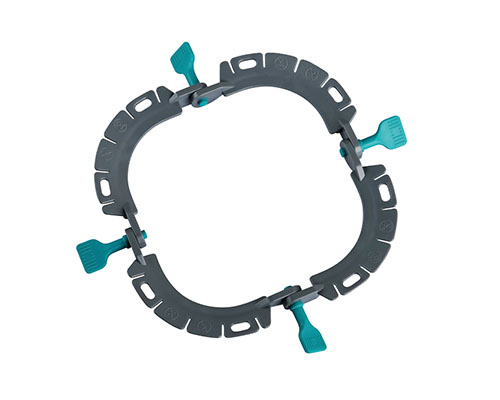

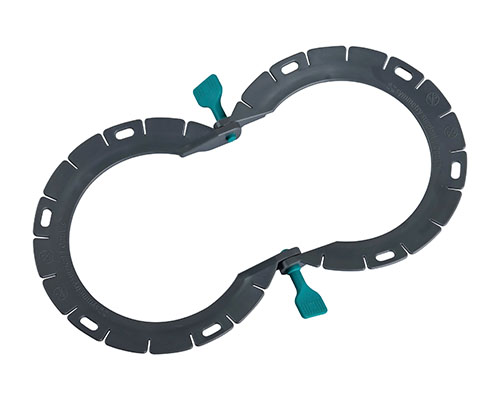







Disposable Skin Retractor Frames
● Design: Ergonomic shapes (Snowman, Square, Horseshoe, etc.) tailored for diverse surgical scenarios.
● Material: High-rigidity medical material with excellent strength to ensure stable and reliable retraction.
● Certifications: CFDA Certified & FDA 510K Exempt; Single-use to prevent cross-infection.
● Application: Suitable for most visualization surgeries, including General, Perineal, and Orthopedic procedures.
Description
Skin Retractor Frames for Surgical Visualization & Incision Retraction
Skin Retractor Frames are designed to support controlled incision retraction and visualization during surgical procedures. This product range includes 8 specialized frame models, allowing surgical teams to select appropriate shapes based on anatomical location and procedural requirements.
Typical Clinical Applications
Designed for visualization-focused surgical environments where stable tissue retraction is required:
- General Surgery
- Perineal Procedures
- Orthopedic Surgery
- Soft Tissue Exposure Procedures
- Open Surgical Fields
- Specialized Visualization Scenarios
Manufactured from high-rigidity medical-grade material, these disposable frames provide reliable structural support during procedures. Multiple ergonomic designs—including Snowman, Square, Horseshoe, and Perineal models— enable flexible configuration across diverse surgical scenarios.
Feature
Key Features
- 8 Specialized Frame Models
Includes Snowman, Square, Horseshoe, Diamond, Figure-8, Rectangle, and Perineal sizes to address different surgical needs. - High-Rigidity Medical Material
Designed with excellent structural strength to support stable incision retraction. - Ergonomic Frame Geometry
Shapes are tailored to anatomical regions and visualization requirements. - Single-Use Design
Disposable configuration supports infection control and operating room hygiene standards. - Regulatory Compliance
CFDA certified and FDA 510K exempt to support regulated market procurement. - OEM / ODM Supply Ready
Suitable for private labeling, bulk supply, and global distributor programs.
Specification
Specifications
| Item No. | Model | Description | Packaging |
|---|---|---|---|
| ASR-G001-000 | Snowman | Blister with paper pack | 50 PCS / CTN |
| ASR-A001-000 | Square | Blister with paper pack | 50 PCS / CTN |
| ASR-H001-000 | Horseshoe | Blister with paper pack | 50 PCS / CTN |
| ASR-C001-000 | Diamond | Blister with paper pack | 50 PCS / CTN |
| ASR-F001-000 | Figure 8 | Blister with paper pack | 50 PCS / CTN |
| ASR-B001-000 | Rectangle | Blister with paper pack | 50 PCS / CTN |
| ASR-E001-000 | Perineal (Small) | Blister with paper pack | 50 PCS / CTN |
| ASR-D001-000 | Perineal (Large) | Blister with paper pack | 50 PCS / CTN |

